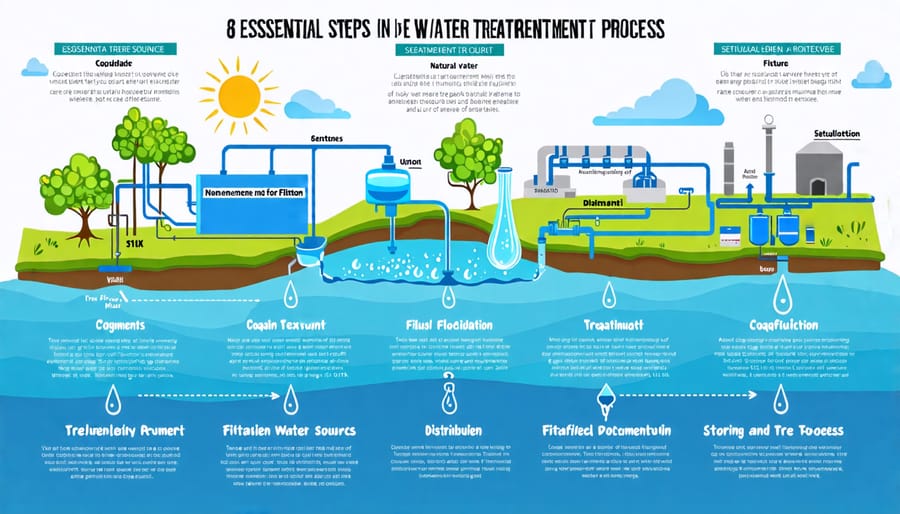
Every day, millions of people turn on their taps, trusting that the water flowing out is safe to drink. But have you ever wondered about the fascinating journey water takes to become clean and potable? From source to faucet, drinking water undergoes a series of crucial treatment steps, each playing a vital role in ensuring the water we consume is free from contaminants and safe for our health.
In this article, we’ll take a deep dive into the science behind drinking water treatment, exploring the essential steps that transform raw water into the crystal-clear liquid we rely on. We’ll break down the process into its key components, explaining how each step works to remove impurities, neutralize harmful microorganisms, and maintain the delicate chemical balance necessary for high-quality drinking water.
By understanding the meticulous care and advanced technologies involved in water treatment, you’ll gain a newfound appreciation for the often-overlooked marvel of clean, readily available tap water. So let’s embark on this eye-opening journey and discover the incredible science that brings us safe drinking water, 24 hours a day, 365 days a year.
(105 words)
Step 1: Water Collection
Water collection is the crucial first step in the drinking water treatment process. Surface water sources like rivers and lakes are common collection points, as well as underground aquifers tapped through wells. According to the U.S. Geological Survey, about 61% of public water supply comes from surface water. Specialized intake structures draw in the raw water, which then passes through coarse screens to remove large debris like leaves, branches, and trash. These screens prevent damage to the treatment equipment downstream. For groundwater, pumps bring the water up from the aquifer to the surface for further treatment. Regardless of the source, this raw water contains various contaminants and requires several more steps before it is safe to drink. But with this initial collection and screening, the journey from source to tap has begun, ensuring a steady supply of water to meet the daily needs of communities.

Step 2: Coagulation and Flocculation
Adding chemicals like alum or iron salts to the water initiates the process of coagulation and flocculation, a crucial step in removing suspended particles. These chemicals, known as coagulants, carry a positive charge that neutralizes the negative charge of the particles, causing them to destabilize and clump together.
As the coagulants interact with the water, they form sticky precipitates that attract the suspended particles, creating larger, heavier clumps called flocs. Gentle mixing helps these flocs grow in size, making them easier to remove in subsequent steps.
The effectiveness of coagulation and flocculation depends on factors like pH, temperature, and the type and dose of coagulants used. Water treatment plant operators carefully monitor and adjust these variables to optimize the process for their specific water source.
By the end of this step, the majority of the suspended particles, including clay, silt, microorganisms, and other organic matter, have been aggregated into flocs that can be readily separated from the water. This sets the stage for the next phase of treatment, where these flocs will be physically removed, bringing the water one step closer to being safe and clean for consumption.
Step 3: Sedimentation
After coagulation and flocculation bind small particles together into larger, heavier flocs, the water flows into sedimentation basins. Here, the force of gravity takes over, allowing the dense flocs to gradually settle to the bottom of the basin while the clearer water remains above. Much like snowfall accumulating on the ground, layer upon layer of floc material builds up, forming a sludge blanket that is periodically removed.
The sedimentation process is a critical step in clarifying the water, as it efficiently separates the majority of suspended solids from the liquid. According to a study published in the Journal of Environmental Engineering, sedimentation can remove up to 90% of the suspended solids in raw water. The clearer water at the top of the basin is then carefully skimmed off and directed to the next stage of treatment, while the sludge undergoes further processing for safe disposal.
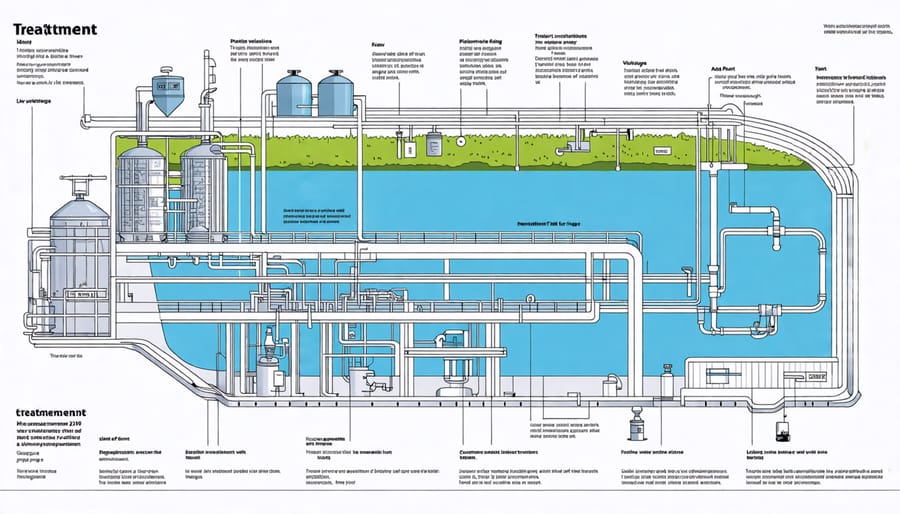
Step 4: Filtration
After coagulation, flocculation, and sedimentation, the water is ready for filtration – a crucial step in removing dissolved particles, chemicals, and microbes. The water passes through multiple layers of filtration materials, typically including sand, gravel, and activated carbon. As the water percolates through these layers, impurities are trapped and removed from the liquid.
The filtration process begins with the water flowing through a bed of sand and gravel. The sand particles act as a sieve, capturing larger suspended solids and particles. The gravel beneath the sand helps to support the sand layer and prevents the filter from clogging. This initial filtration stage removes a significant portion of the remaining impurities.
Next, the water moves through a layer of activated carbon, often in the form of granular activated carbon (GAC). GAC is a highly porous material with a large surface area, making it an effective adsorbent. As the water passes through the GAC, dissolved organic compounds, chemicals, and other contaminants are attracted to and trapped within the pores of the carbon. This process helps to remove unpleasant tastes, odors, and colors from the water.
In some treatment plants, additional filtration methods may be employed, such as membrane filtration. Membrane filters have tiny pores that can remove even smaller particles and microorganisms, including bacteria and protozoa. These advanced filtration techniques ensure that the water is thoroughly cleansed and safe for consumption.
Throughout the filtration process, the water quality is continually monitored to ensure that the filters are functioning properly and that the treated water meets all necessary standards. Regular maintenance, such as backwashing the filters to remove accumulated debris, is essential to keep the filtration system operating at optimal efficiency.
By the end of the filtration stage, the water is clear, clean, and free from the vast majority of contaminants. This multi-stage filtration process is a testament to the ingenuity and scientific understanding that goes into ensuring the safety and purity of our drinking water.
Step 5: Disinfection
After the filtration process removes the majority of contaminants, disinfection is the critical final step in drinking water treatment to ensure the water is safe for consumption. Chlorination is the most common disinfection method, where chlorine is added to the water to kill any remaining harmful microorganisms like bacteria, viruses, and protozoa. Chlorine is highly effective at eliminating waterborne pathogens and provides residual protection as the water travels through distribution pipes to prevent regrowth of microbes.
An alternative disinfection method is ultraviolet (UV) light, which inactivates pathogens by damaging their DNA. While UV light doesn’t provide residual disinfection, it avoids the potential formation of disinfection byproducts associated with chlorine. Ongoing research explores innovative ways to harness renewable energy sources like solar power to create clean drinking water in a sustainable manner.
Disinfection is a crucial safeguard in multi-barrier water treatment. As Dr. Lisa Daniels from the Water Research Foundation explains, “Disinfection is the key step that prevents waterborne diseases and ensures public health. It’s the last line of defense before the water reaches our taps.” Rigorous monitoring and testing confirm disinfection effectiveness and verify the treated water meets all regulatory standards before distribution to homes and businesses. This step gives consumers confidence that every glass of tap water is clean and safe to drink.
Step 6: Storage and Distribution
After the drinking water has undergone rigorous treatment processes, it is ready for storage and distribution. The treated water is stored in secure, closed tanks or reservoirs to protect it from contamination. These storage facilities are designed to maintain the water’s quality and ensure a steady supply for the community.
From the storage tanks, the water enters a complex network of pipes that distribute it to homes, businesses, and public facilities. This distribution system is carefully engineered to deliver water at the right pressure and flow rate. Regular maintenance and monitoring of the pipes ensure that the water remains safe and free from contaminants as it travels to the tap.
Water utilities work round the clock to ensure that the storage and distribution infrastructure is reliable and efficient. By investing in modern technology and best practices, they strive to provide consumers with a dependable supply of clean, treated drinking water whenever they turn on the faucet.
Conclusion
The multi-step drinking water treatment process is a remarkable feat of science and engineering that transforms raw water from various sources into safe, potable drinking water for our communities. From initial screening and coagulation to sedimentation, filtration, and disinfection, each step plays a critical role in removing contaminants and ensuring the water meets strict quality standards. Ongoing testing and monitoring throughout the process and distribution system maintain this high level of water quality and safety. The end result is clean, healthy drinking water flowing from our taps, thanks to the diligent efforts of water treatment professionals. As technology advances, innovative solutions like solar-powered water desalination offer promising ways to expand access to safe drinking water sustainably.